find the electron configuration ti|Electron configuration of titanium : iloilo To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number. Lottery Licence #AGD-338861, 68, 69-24 ™QEII Home Lottery and Cash Calendar are trademarks. ®50/50 Add-On and 100 Days of Winning are registered trademarks. QEII Health Sciences Foundation | Charitable Registration: #88646 3496 RR0001 Prizes awarded may not be exactly as illustrated.
find the electron configuration ti,To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number.
find the electron configuration ti "Ti"^(2+): ["Ar"]3d^2 A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom. In this case, titanium, "Ti", is located in period .
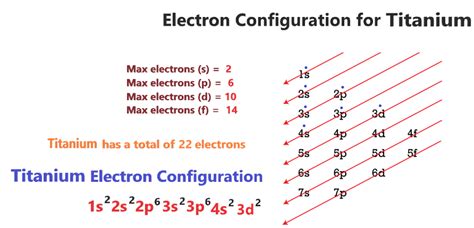
The full electron configuration of titanium is 1s2 2s2 2p6 3s2 3p6 4s2 3d2 and the abbreviated electron configuration is [Ar]3d24s2. With the Electron configuration of each .
Electron configuration of titanium Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and high . The electronic configuration of an element can be drawn by shell wise and subshell wise. Let us look into the following steps. Writing the number of shells involved .Titanium electron configuration. ← Electronic configurations of elements. Ti (Titanium) is an element with position number 22 in the periodic table. Located in the IV period. . This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble.Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and Tit. | Channels for Pearson+. Next video. General Chemistry 10. Periodic Properties of the Elements The Electron Configuration: Ions. 3m.
The electronic configuration of an atom describes how many electrons the atom has and how these electrons are arranged into different electron shells and subshells. The atomic number of titanium is 22, which means that a titanium atom has a total of 22 electrons. Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Titanium .

Video Transcript. Which of the following is the electronic configuration of Ti? (A) Ar 4s1 3d3, (B) Ar 3s2 4d2, (C) Kr 4s2 3d2, (D) Kr 5s2 4d2, (E) Ar 4s2 3d2. To solve this problem, we need to select the answer choice that shows the correct electron . To find the number of valence electrons for Titanium (Ti) we need to look at its electron configuration. This is necessary because Ti is a transition metal .
If we lose two electrons, we have a net deposited two charge. We form the calcium to ion. The two electrons that we would lose to form the calcium two plus ion are these. These two .
In this lesson, students will explore an interactive periodic table, using sliders to move around the table. The electron configuration of the element is displayed graphically. The electron configuration is also displayed in the standard textual form.The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a . The electron configuration for Titanium ion (Ti 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6. The number of valence electrons available for the Titanium atom is 4. Titanium is situated in the transition metal group and has an atomic number of 22. The orbital diagram for Titanium is drawn by following three principles – the Aufbau principle, Hund’s .In several cases, the ground state electron configurations are different from those predicted by Figure 6.8.1 6.8. 1. Some of these anomalies occur as the 3 d orbitals are filled. For example, the observed ground state electron configuration of chromium is [Ar]4 s1 3 d5 rather than the predicted [Ar]4 s2 3 d4.

Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle .That is, recognizing that each .
Table 0.1.2.1.1. Four considerations in predicting ground state electron configuration of multi-electron atoms and ions. (1) Electrons will occupy the lowest energy orbitals in order to minimize the total energy. The two quantum numbers that are related to energy in multi-electron atoms are n, and l. Thus, orbitals with the lowest values of n . This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble. To write the orbital diagram for the Titanium (Ti) first we need to write the electron configuration for just Ti. To do that we need to find the number of e.
Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation .find the electron configuration ti Electron configuration of titaniumAnswer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled.
The easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. It looks something like this.
Electron configurations help you to do this. To calculate an electron configuration, divide the periodic table into sections to represent the atomic orbitals, the regions where electrons are contained. Groups one and two are the s-block, three through 12 represent the d-block, 13 to 18 are the p-block and the two rows at the bottom are the . Electron configurations of ions. To find the electron configuration for an ion, first identify the configuration for the neutral atom. Then, add or remove electrons depending on the ion's charge. For example, to find the configuration for the lithium ion (Li⁺), start with neutral lithium (1s²2s¹). Then, since the lithium ion has one less .
Solution: Method 2. Locate the atom on the periodic table. Figure 1.9.1 1.9. 1: Periodic table of the elements with the location of vanadium (V) highlighted. (CC-BY-NC-SA; Kathryn A. Newton) Starting at hydrogen and the 1s subshell, read across each row of the periodic table until you get to your chosen element.
find the electron configuration ti|Electron configuration of titanium
PH0 · What is the electron configuration of #"Ti"^(2+)#?
PH1 · Titanium Electron Configuration:(Explained for Beginners)
PH2 · Titanium Electron Configuration (Ti) with Orbital Diagram
PH3 · Orbital Diagram of Titanium (Ti), electron configuration, and
PH4 · Electron configuration of titanium
PH5 · Electron configuration for Titanium (element 22). Orbital diagram
PH6 · Electron Configuration for Titanium (Ti2+,Ti3+,Ti4+ ions)
PH7 · Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and
PH8 · Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and
PH9 · Electron Configuration for Ti , Ti3+, and Ti4
PH10 · Determining the Electronic Configuration of Titanium